End-to-End Scientific Expertise

Data-Driven & Accurate Solutions

Validation-Ready Approach

Faster Decisions, Reduced Risk
About Us
From Selection to Validation — Precision at Every Step.
ALTIUS Advisory is a specialized consulting partner dedicated to enabling the smooth and compliant implementation of FT-NIR (Fourier Transform Near-Infrared) technologies across the pharmaceutical lifecycle. Formed by a team of analytical, regulatory, and process experts, ALTIUS helps organizations harness the power of FT-NIR for data-driven quality control and process understanding. We guide our clients through every stage of FT-NIR deployment—from feasibility assessment and method development to regulatory approval and commercial release. Our approach ensures that each model is scientifically justified, robust, and aligned with global regulatory expectations.
Altius Advisory
Services
Altius Advisory
Approvals
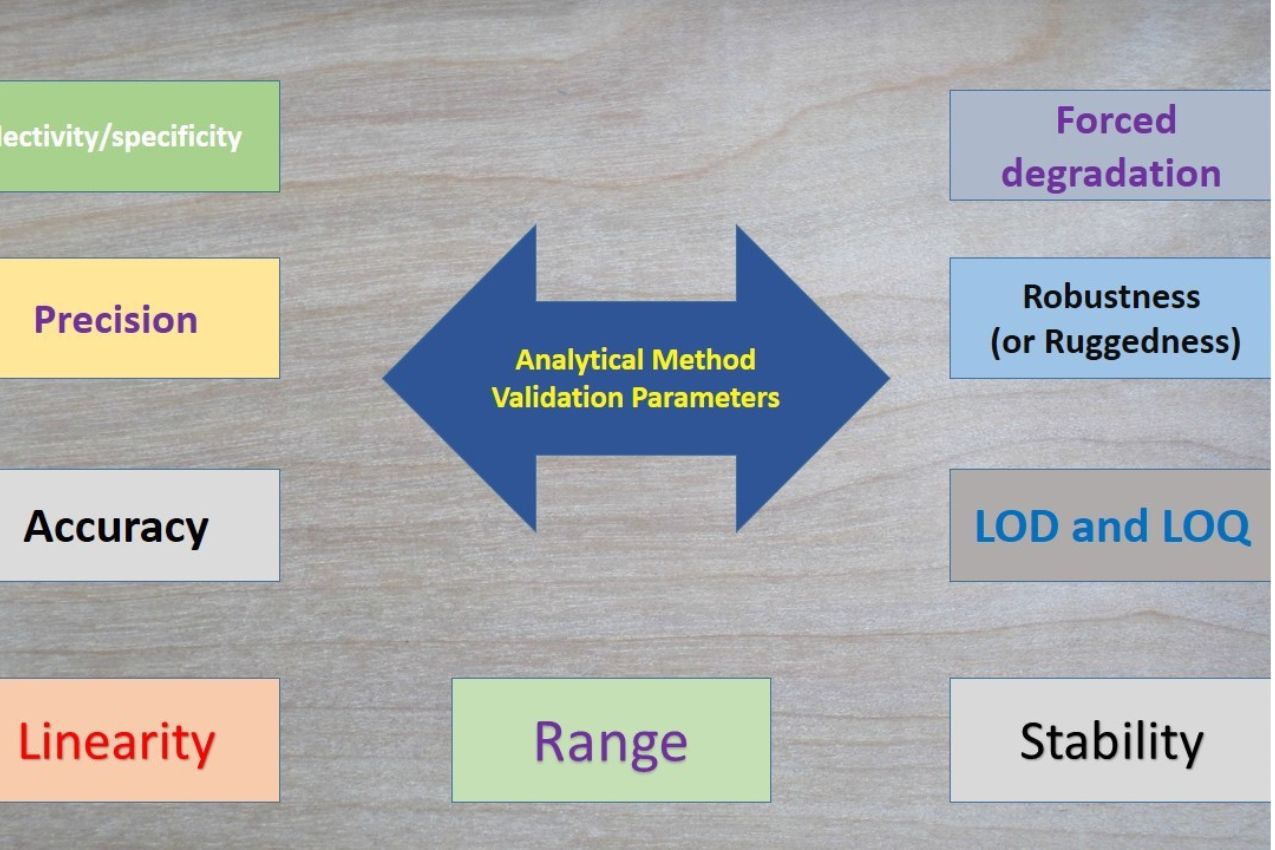
Altius Advisory provides comprehensive guidance for the preparation and submission of complete and compliant documentation for NIR methods, ensuring alignment with current regulatory expectations and smooth approval processes.
Implementation
Altius Advisory partners closely with R&D, QC, QA and Manufacturing QMS to ensure a seamless transition from NIR method development to full-scale implementation. Our collaborative approach bridges the gap between laboratory innovation and commercial deployment, enabling regulatory readiness and smooth market release.
Support
Altius Advisory enables organizations to implement a sustainable lifecycle management strategy for NIR methods, supporting continuous improvement, regulatory compliance, and operational excellence.